your technical files
with just one webapp
With the power of MDGest, you can now focus only on functionaries for your Medical Device, while leaving the contents and deadlines management on software!
Demo

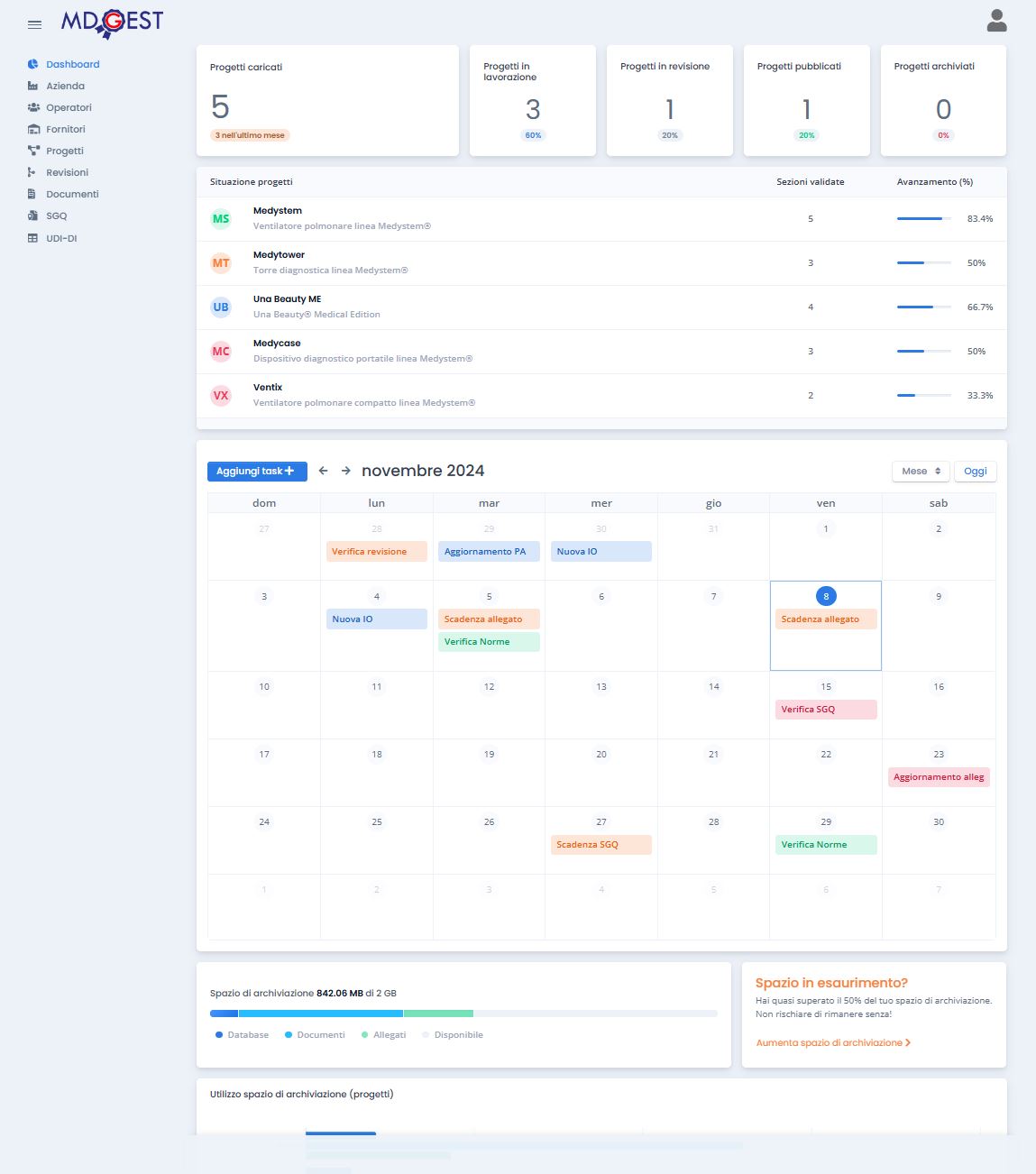
WebApp for Medical Device documentation management
According with MDR – UE 2017/745 and his updates and Annex II scheme for technical documentation to submit at Notified Body.
MANAGE
Manage the deadline
Manage all the documentation of the medical device technical file in one place with the management of the deadlines of all documents.
BUILD
Build medical file
Thanks to the fields imposed by All.II, the process of building the medical file is guided and simplified, preventing the risk to forget something.
DEPLOY
Ready for Submission
Once all the information has been entered, it will be possible to export the documents in different formats, ready for submission to the notified body, for analysis to Annex IX-X-XI.
RESERVED
Remote access
Special accounts and specific access area for review of notified body assessment of technical documentation and clinical evaluation documentation.
Here's what's in it for you
Things you will get right out of the box with MD Gest.
Manage Annex
Technical file project management. Attach documents, images and drawings to your projects. Choose the size of the storage space according to your needs.
Manage UDI-DI
Manage your UDI-DI codes. Import codes issued directly from files received from Issues Agencies. Keep track of codes already used and those that are still free.
Manage multiple technical files
Multiple technical technical files are managed with the same platform, using common data like ISO 13485 procedures, key persons, suppliers, sites of production and many more re-usable objects.
Vigilance and Surveillance
According with MDR, for each device, manufacturers manage a complete flow of document, implementation, maintain and update a post-market surveillance system in a manner that is proportionate to the risk class and appropriate for the type of device. That system shall be an integral part of the manufacturer's quality management system. According to Article 83, the software permits to create a post-market surveillance system shall be suited to actively and systematically gathering, recording and analysing relevant data on the quality, performance and safety of a device throughout its entire lifetime, and to drawing the necessary conclusions and to determining, implementing and monitoring any preventive and corrective actions.
Our Mission
MD Gest is a WebApp for a complete Medical Device Technical Files management.Its schemas are tailored on MDF 745/17 UE and his updates. With the platform you will be able to manage all Medical Device of your company in only one place and you can have under control according deadlines, updates, and PMS-PSUR according to Meddev and MDCG.
MDGest è una WebApplication prodotta da Fatromed Srl - 2024 ©
v3.21.1